The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or to view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu. If you haven't clicked in yet, please pay attention to the clicker question. I'll give you a little bit more time to click in and I'll give you a 10-second warning. Alright, let's go ahead and take 10 more seconds. Does someone want to say how they got this one right? Anyone willing to say how they got it right? We have American Chemical Society whatever these are called that hang around your neck and clip things to them. Yes, there's the word. Oh, there we go. Okay, so this molecule is phosphorus. It has five valence electrons and each hydrogen has three. So, it's a total of eight. It has three bonding and then one lone pair which makes it a tetrahedral. But then the lone pair pushes the other bonds, so it's less than 109.5 degrees. Excellent. Yeah, I see that some people just decided that there were three things bound to it and so then they decided either 120 or less than 120. But you really need to do the Lewis structure and see how many lone pairs there are first. So, once you do the Lewis structure, then you figure out the parent geometry, which in this case is tetrahedral. And we're going to have more practice on these coming up. Alright, so we're continuing on now with molecular orbital theory. We started the course talking about atomic structure and then we talked about atomic orbitals or wave functions. And then we moved on to bonding, that atoms can come together and...
Award-winning PDF software





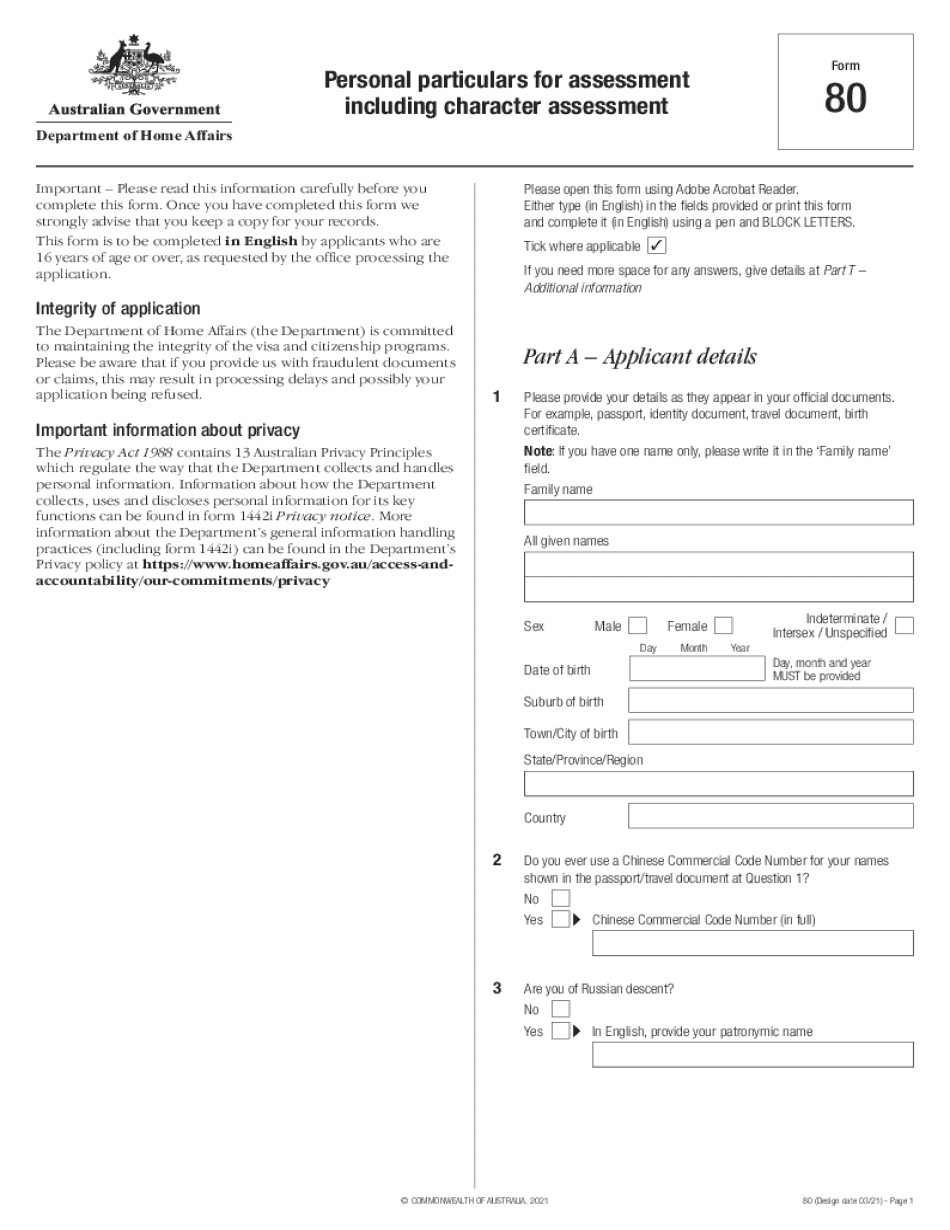
1221 question 34 Form: What You Should Know
Form 1221 — Additional Personal Particulars Form Australia — the section for You MUST have completed it and signed the paper, and — the section for YOU MUST have completed it and signed the paper, and — the section for the must also have provided a copy of a current passport or other acceptable proof of identity as to what your country of residence is (and not what your name is) and is able to provide a passport (this is a bit of a limitation on where you can actually move to, don't worry.) You must have completed the section for you MUST have provided a passport and is able to provide a passport (this is a bit of a limitation on where you can actually move to, don't worry.) This section will ask you for any information relevant to the section in the Form 1221. It contains the information to answer questions that the interviewer/office-worker is searching for. Here's how that section looks like : Step Form 1221 — Additional Personal Particulars Form Australia How to Form 1221 How to Fill This includes your comments on your questions also as I answer based YouTube · Helping · Jul 5, 2017 Visa 485 Form 1221 — Optional Section — Optional part of form that shows what you did with the additional personal details form. Form 1221 — Additional Personal Particulars Form Australia How to Fill This includes your comments on your questions also as I answer based YouTube · Helping · Dec 6, 2016 Form 1221 Help — Expat Forum Feb 7, 2025 — I am an Australian citizen and now residing in Hong Kong. I have submitted the Additional Personal Particulars Form for a (Visa 485) for my partner, who has inherited my Australian passports (Australian and Hong Kong) and has a Hong Kong Passport (as does my sister who has married an Australian). Now I have received a confirmation letter of the application from the Australian authorities (I have the letter with me) and a second letter from the Hong Kong authorities, confirming that the additional personal details form had been filled out correctly (as requested). The application has not yet been processed. The only information we have for now is a notification of completion on the (Visa 485) Form 1.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do Form 80, steer clear of blunders along with furnish it in a timely manner:
How to complete any Form 80 Online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your Form 80 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your Form 80 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Form 1221 question 34